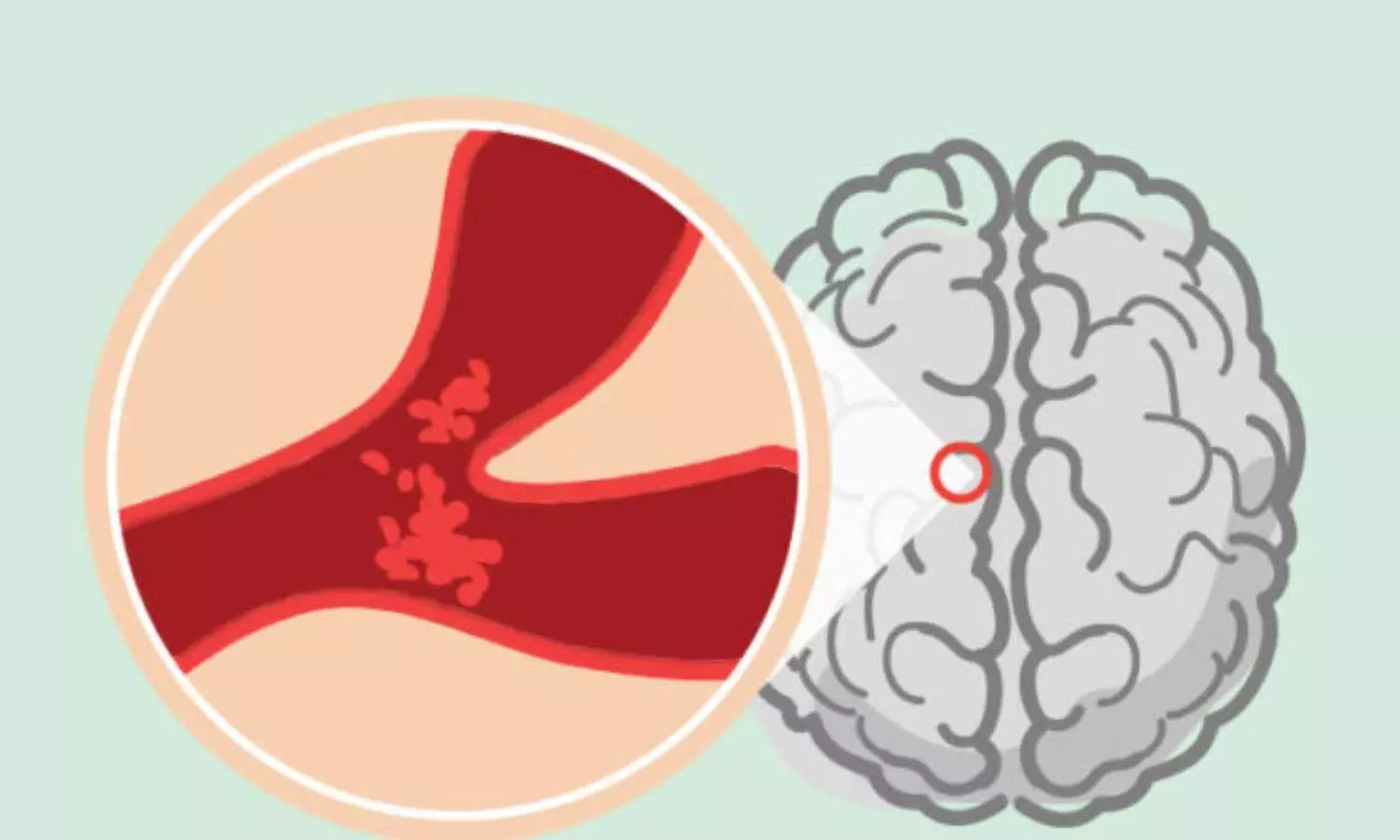
A groundbreaking clinical trial conducted by UCLA Health researchers has demonstrated the potential of high-definition cathodal transcranial direct current stimulation (HD C-tDCS) as a new treatment for the most common type of stroke. The pilot study found that HD C-tDCS showed good tolerability and indicated favorable effects on the rescue of threatened brain tissue. The noninvasive treatment was initiated within a median of 12.5 minutes of randomization in the final enrolled patients. This study is the first of its kind in humans to investigate the feasibility of using targeted electrical current to treat acute ischemic stroke. Acute ischemic stroke occurs when a clot obstructs blood flow to a region of the brain and accounts for approximately 85% of all strokes. Stroke is a leading cause of death and disability in the United States.
The current treatments available for acute ischemic stroke, such as clot-dissolving drugs and clot removal devices, are not suitable for many patients. Even among those eligible for these treatments, around 20-30% are left with long-term disabilities. In this new study, published in JAMA Network Open, researchers at UCLA Health tested HD C-tDCS as an innovative therapy for acute ischemic stroke. This involved strategically placing electrodes on the scalp to deliver a weak inhibitory electrical current to the part of the brain with reduced blood flow. This form of noninvasive stimulation has been used to treat various neurological and psychiatric conditions, and previous studies have suggested that it may affect brain blood flow.
The researchers hypothesized that HD C-tDCS could potentially enhance blood flow to the brain regions impacted by stroke and protect the penumbra, which refers to the threatened brain tissue surrounding the core of the stroke, from irreversible damage. The pilot study involved 10 acute stroke patients who were ineligible for existing treatments and within 24 hours of stroke onset. Seven patients received active HD C-tDCS treatment, while three received sham stimulation. Using brain scans that acute stroke patients typically undergo upon arrival, the researchers were able to identify the area with reduced blood flow and target the HD C-tDCS treatment to that region.
Lead researcher, Dr. Mersedeh Bahr-Hosseini, a vascular neurologist at UCLA Health, explained that the treatment was designed to be as targeted and individualized as possible, focusing solely on the brain area with low blood flow or affected by the stroke. The first group of patients received 20 minutes of 1 milliamp of stimulation, while the remaining patients received an escalated dose of 2 milliamps for 20 minutes. The researchers successfully administered the treatment in emergency settings, and the patients tolerated it well.
The most exciting finding from the study was that in patients who received HD C-tDCS, a median of 66% of the penumbra – the threatened brain tissue surrounding the core of the stroke – was rescued within the first 24 hours after the stroke, compared to 0% in the sham group. This suggests that HD C-tDCS has the potential to significantly improve outcomes for acute ischemic stroke patients.
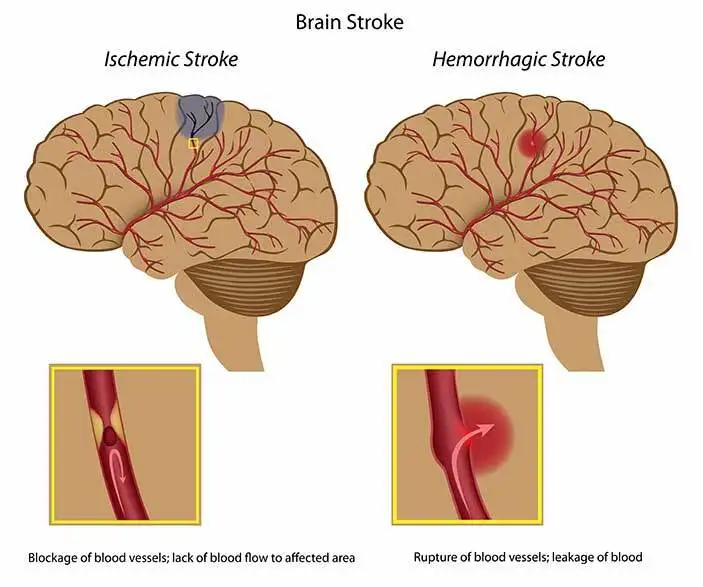
The hemodynamic brain scans conducted immediately after the treatment revealed that patients who received HD C-tDCS experienced improved blood flow. The improvement was more prominent in patients who received a dosage of two milliamps compared to those who received a dosage of one milliamp. On the other hand, the sham group showed a decrease in blood flow. Dr. Bahr-Hosseini described this finding as exciting because it suggests a potential true biological effect of the treatment.
To gather more data on the safety and effectiveness of HD C-tDCS, researchers are planning a new multi-site study in collaboration with Johns Hopkins, Duke University, and the University of Pennsylvania. The upcoming study will include not only patients who are ineligible for clot-dissolving drugs but also patients who can receive intravenous thrombolytics.